 |
 |
 |
Welcome to BioSciCon Companies |
||
|
|||||||
BioSciCon, the Biomedical Science Consulting Company, Inc. is a woman-owned, biotechnology R&D, small business company located in Rockville, Maryland, USA. Incorporated in 1996, BioSciCon's primary activities have been focused on the development of a proprietary platform MarkPap® technology for improving Pap test and a more accurate early detection of cervical cancer and precancerosis. BioSciCon is a R&D component of a consortium with four more companies: MarkPap LLC, a company with a mission to commercialize BioSciCon's products as they emerge from R&D world-wide, MarkPap Pacific LLC with a limited mission to promote MarkPap technology in China, MarkPap India, LLC for India, and The Global Academy for Women's Health, Inc., an independent non-profit organization which integrates prior non-profit activities of BioSciCon's founders continuing their legacy - to work for public benefit. BioSciCon designs and develops a proprietary platform technology under the brand name MarkPap® which is biomarker-based and telemedicine-empowered. The products in the production pipeline are designed to be low-cost, simple, fast, accurate, accessible, equitable and infrastructure-independent with selective intended application, such as Mass Pap Smear Cervical Cancer Screening World-Wide (48, 50). This is an emerging biomarker-based technology for the enhancement of the visibility of abnormal cells on Pap specimens (1,9). The biomarker is cytoplasmic expression of molecular changes occurring in the nuclei of epithelial cell undergoing transformation from normal to dysplastic and malignant. When visualized with MarkPap® technology, the biomarker, an aggregate of nanoparticles of chromogen precipitating in time in the cytoplasm, appears as a red pigment on the Papanicolaou bluish background in abnormal cells serving as their locator (see images bellow and galleries). This is a cytoplasmic protein biomarker distinct from nuclear features commonly used for cytological screening and, therefore, it is an addition to the standard criteria for reading and interpretation of cervical specimens (see images).The biomarker appears exclusively in dysplastic, cancerous and HPV affected cervical cells. Normal squamous epithelial cells are entirely negative. Since the biomarker is positive in HPV disease (highlighting koilocytes), the test may become a new cytological tool for detecting epithelial tissue response to HPV infection and for monitoring of the effect of the new HPV vaccines (20,24,32). Based on the results from clinical-laboratory trials on 2,000 subjects/specimens (general population and women at high risk), MarkPap® test proved to be superior (better diagnostic accuracy), more productive (faster screening), better controlled (control slides), customer friendly (MarkPap® kit), and less expensive than conventional and liquid-based Pap test (9,16,19). MarkPap® test has reduced the false negative rates of cervical cancer screening to less then 5% (8,9). MarkPap® products will become available for marketing in the USA after FDA approval and worldwide after the approval of local governments. The presence of the biomarker brings the opportunity for utilizing this technology in telemedicine. Our new product MarkPap® Digital is designed for telecytopathology --diagnosis on distance. (23,25,26,27,36,39,40,45-47). Capturing images at the point-of-care could be done with digital camera or cellphone camera by a low-trained person who should select only "red" biomarker positive cells.
MarkPap® Images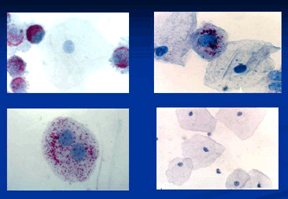 Click on the image for a larger view Legend: Upper left: COMBO Control Slide. Central marker negative (blue) normal epithelial cell surrounded with marker positive (red) HeLa cultured cervical carcinoma cells. Upper right: Cervical specimen with two negative normal squamous epithelial cells, and one small abnormal positive cell. Lower left: HPV infected binuclear positive cell. Lower right: Normal cervico-vaginal specimen, with several normal marker negative squamous epithelial cells. Only abnormal cells are highlighted by the red biomarker. Normal cells are entirely negative (blue). This is a red "stop light" for the screener. Hard to miss marked cells! |
|